Translate this page into:
Positron emission tomography computed tomography in the diagnosis of prostate cancer
*Corresponding author: Rashmi Umesh Angadi, Department of Nuclear Medicine and Molecular Diagnostics, Neumod, Hubli, Karnataka, India. doctor.rashmi@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Angadi RU. Positron emission tomography computed tomography in the diagnosis of prostate cancer. Karnataka Med J. 2024;47:15-24. doi: 10.25259/KMJ_4_2023
Abstract
Prostate cancer (PCa) is one of the most frequent cancers in men and constitutes the third most common cause of cancer deaths. Early diagnosis of primary PCa, accurate staging, as well as accurate restaging in the case of cancer recurrence after primary treatment are important for delivering the appropriate therapy. In the past 10 years, functional and molecular imaging by means of positron emission tomography/computed tomography (PET/CT) and PET/magnetic resonance are increasingly being used for such indications. This article provides a radiolabelled tracer-based review of the diagnostic value of PET/CT in primary and recurrent PCa.
Keywords
Prostate cancer
Positron emission tomography
Staging
Recurrence
Prostate-specific membrane antigen
INTRODUCTION
Prostate cancer (PCa) is currently the most prevalent form of cancer in men (301,500 cases, 24.1% of all incident cases) and constitutes the third most common cause of cancer deaths (10.4%) in the European Union.[1] The main modalities to diagnose PCa include a digital rectal examination, serum concentration of prostate-specific antigen (PSA) and transrectal ultrasound (TRUS)-guided biopsies.[2] For imaging patients with PCa, several conventional imaging modalities are available: TRUS, computed tomography (CT), magnetic resonance imaging (MRI) and bone scintigraphy. Morphological imaging techniques, such as CT, MRI and TRUS, have only shown a limited accuracy for primary diagnosis of PCa, recurrent disease as well as advanced disease. Patients suffering from PCa can be categorised into three groups with respect to disease onset and course of disease; patients with primary PCa who have localised disease, patients with a biochemical recurrence (BCR) after primary treatment and patients with advanced stages of disease. Concerning diagnosis of primary PCa, CT is limited as intra-prostatic cancer tissue does not have a different density compared with normal prostate tissue or benign prostate pathologies. The detection of lymph node metastases is limited in morphological imaging (CT and MRI), both methods rely mainly on the size criterion; however, lymph nodes smaller than 1 cm can also bear tumour metastasis. Concerning bone metastases, CT can be used for imaging trabecular changes caused by the metastasis; however, those changes appear relatively late in the course of bone metastases.
PCa mortality has not decreased significantly, and about 15–25% of cases still experience biochemical failure or BCR, following primary definitive therapy[3-9] despite the high success rates of primary definitive therapy options, including radiation therapy (RT) and radical prostatectomy (RP). Therefore, positron emission tomography (PET) imaging in PCa has an emerging role to play,[10] as it provides more functional information than other imaging modalities and can detect metastases. In PET, radiolabelled biomolecules pertinent to cellular processes are used to detect metabolic activity or cell surface molecules that are usually associated with cancer.
PET is also useful for identifying patients suitable for active surveillance for accurate pre-RP/pre-RT staging. PET imaging is usually combined with CT or MRI which improves anatomic localisation of any abnormal tracer uptake. Furthermore, CT or MRI helps differentiate physiologic activity (e.g., activity in the ureters) from pathologic uptake.[11] Clinical experience with PET in PCa is increasing. However, the increased costs of PET compared with other imaging methods mean that the choice of PET in an imaging algorithm must be judiciously considered.
PET agent, 18F-fluorodeoxyglucose (18F-FDG) was introduced as a routine clinical imaging method in the early 2000s. It has been the mainstay of clinical molecular imaging in cancer. However, PCas are not particularly avid for 18F-FDG. Recently, several newer tracers have been introduced. They are able to target a variety of metabolites (e.g. glucose, fatty acids and amino acids), antigens (e.g. prostate-specific membrane antigen and prostate-specific stem cell antigen), angiogenesis, hypoxia and gene-based pathways[10] [Figure 1]. Still, very few of these agents are available clinically and even fewer are reimbursed.[12] The most common PET radiotracers in the imaging evaluation of PCa are summarised in Table 1.
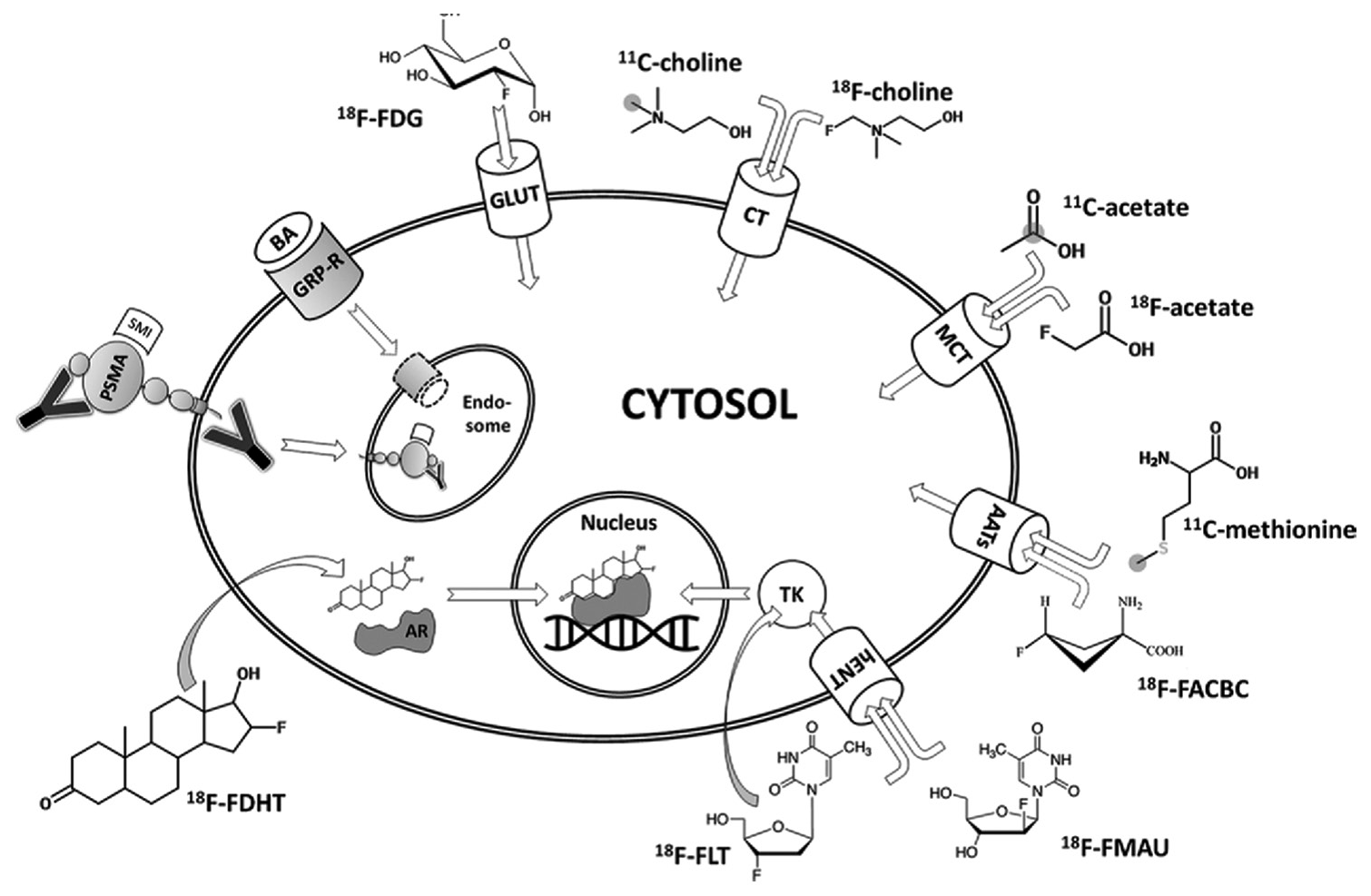
- Diagram of an overview of molecular imaging strategies currently applied for prostate cancer. Grey circles indicate the position of carbon 11 (11C) atoms. Curved arrows indicate free transmembranous diffusion. AATs: Amino acid transporters, AR: Androgen receptor, BA: Bombesin analogue, CT: Choline transporter, 18F-FACBC: Anti-fluorine 18 (18F)-1-amino-3-fluorocyclobutane-1-carboxylic acid, 18F-FDG: 18F-fluorodeoxyglucose, 18F-FDHT: 18F-16b-fluoro-5adihydrotestosterone, 18F-FLT: 18F-fluorothymidine, 18F-FMAU: 18F-fluoro-methyl-arabinofuranosyluracil, GLUT: Glucose transporter, GRP-R: Gastrin-releasing peptide receptor, hENT: Human equilibrative nucleoside transporter, MCT: Monocarboxylate transporter, PSMA: Prostate-specific membrane antigen, SMI: Small molecule inhibitor, TK: Thymidine kinase, Y: Antibody.
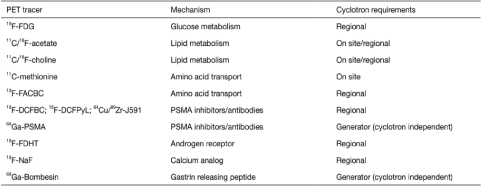 |
18F: Fluorine 18, 11C: Carbon 11, 68Ga: Gallium 68, PSMA: Prostate specific membrane antigen, FDHT: Flurodihydrotestosterone, NaF: Sodium Fluoride, FDG: Fluorodeoxyglucose
18F-FDG
FDG is a glucose analogue with the replacement of the oxygen in the C-2 position with 18f. 18F has a half-life of 110 min, making it ideal for clinical use and 18F also has favourable imaging characteristics. Its uptake is elevated in most primary and metastatic cancers due to malignancy related increased glucose uptake due to Warburg physiology or aerobic glycolysis that is inherently less efficient than oxidative phosphorylation.[13] However, its excretion into the urinary system poses a problem for pelvic malignancies. Although 18F-FDG shows an excellent performance in many malignant lesions, in PCa, this tracer is limited due to generally low glucose utilisation in PCa cells.[14-17] Moreover, in 18F-FDG PET/CT studies, the tracer showed overlapping uptake in normal, benign and malignant tissues, resulting in poor specificity.[18-20] However, evidence for its value in the initial staging of PCa remains scant, and it is not recommended.[21,22]
18F-FDG PET/CT has low specificity for PCa, despite its wide availability and use in cancer imaging, and consequently, its use is reserved for late-stage metastatic disease. A recent systematic review and meta-analysis of 3586 men with PCa compared the diagnostic accuracy among four PET/CT radiotracers (18F-FDG, 11C-choline, 18F-choline and 11C-acetate) suggesting diagnostic superiority of 18F-choline, ranked as the most favourable with the highest value of area under curve (AUC) (AUC = 0.94; 95% confidence interval [CI]: 0.92–0.96), whereas 18F-FDG was the least favourable (AUC = 0.73; 95% CI: 0.69–0.77) for PCa detection in a recent systematic review and meta-analysis of 3,586 men with PCa.
11C/18F-CHOLINE
Choline is essential for the synthesis of cell membranes during cell growth. Second, by way of its metabolite betaine, choline participates in the synthesis of methionine; and third, choline is the biochemical originator of the neurotransmitter acetylcholine. Because choline is a charged hydrophilic cation, it requires specific membrane transporters to enter cells. Several mechanisms have been shown to be responsible for the accumulation of choline and its metabolites in cancer cells. 11C-labelled choline has a short half-life (20 min) and is primarily excreted through the hepatobiliary system with only little urinary excretion, which is advantageous for the evaluation of the prostate gland. 18F-fluorocholine is excreted by the urinary tract, leading to a higher accumulation of the tracer in the bladder, which is less favourable for PCa imaging. However, 18F-fluorocholine has a longer half-life (110 min), which makes it more practical.
In primary disease, specificity is reduced by the high uptake of these agents in BPH and other benign conditions. However, 11C-/18F-cholines are useful in patients with suspected recurrence after first line or salvage therapies, and for that reason, the Food and Drug Administration (FDA) approved the use of 11C-choline for BCR evaluation in September 2012 [Figure 2]. In the results of a meta-analysis on this topic, investigators reported pooled sensitivity and pooled specificity of 86% and 93%, respectively, for all sites of disease (area under the receiver operating characteristic curve, 0.949).
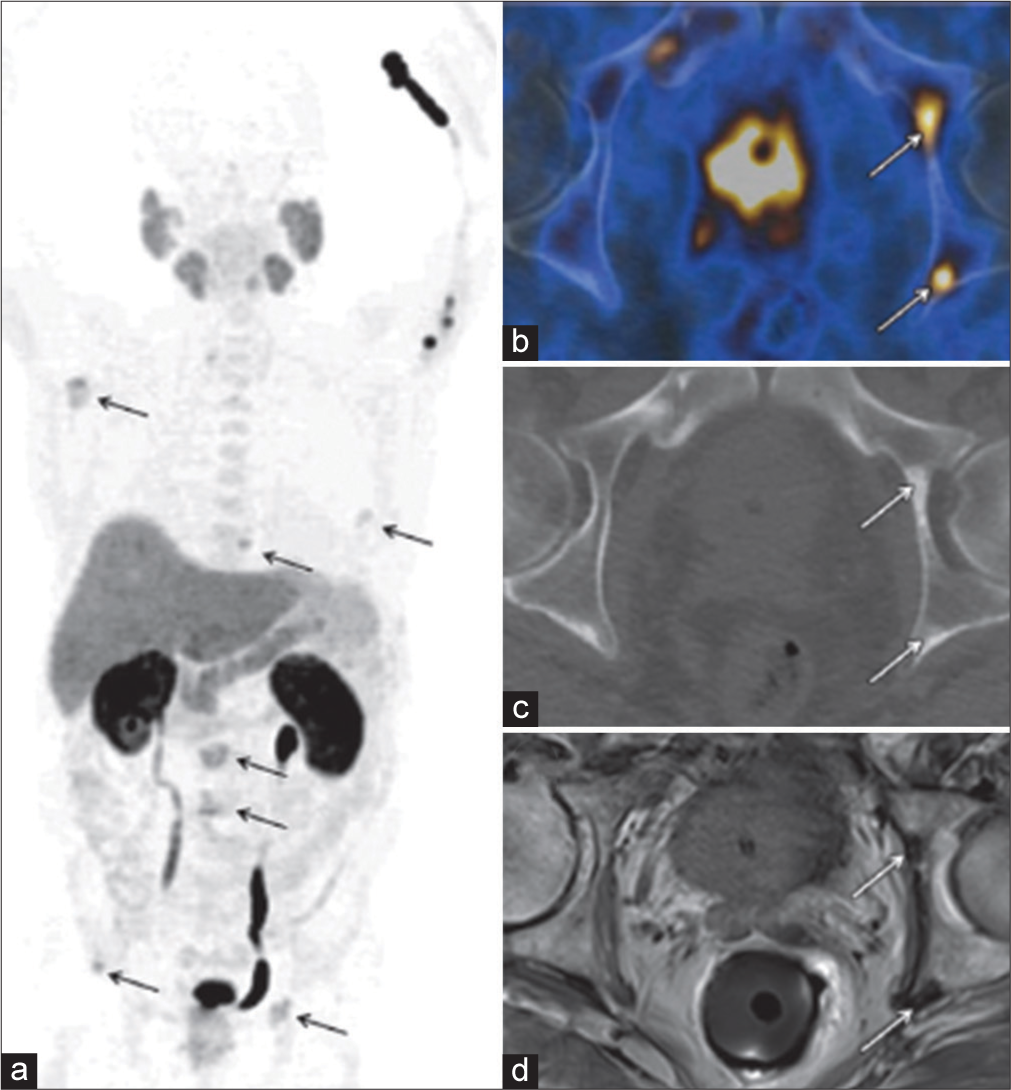
-
18F-Choline PET/CT and MR imaging of a 72-year-old man with newly diagnosed prostate cancer (Gleason score, 5+5 =10). (a) Late coronal maximum intensity projection image from 18F-Choline PET/CT shows focal tracer uptake in multiple bone lesions (arrows). (b) Axial fused PET/CT image of the pelvis depicts intense focal tracer uptake (arrows) in the left acetabulum, which helps confirm the findings in (a). (c) Axial CT image shows minimal sclerotic changes (arrows) in the left acetabulum. (d) Corresponding axial T1-weighted MR image shows minimal hypointense changes (arrows) in the left acetabulum. PET/CT: Positron emission tomography/computed tomography, MR: Magnetic resonance.
11C/18F-ACETATE
Acetate is a vital part of fatty acid metabolism. It is converted by Acetyl-CoA synthetase to Acetyl-CoA, which is further converted by fatty acid synthetase (FAS) into fatty acids, which are then incorporated into the cellular membrane. In human cancer cells, several enzymatic pathways of acetate processing were observed to be upregulated or essential for cell survival.[17-19] In PCa cells, the upregulation of FAS was found to be the most likely molecular basis for the increased cellular uptake of acetate[20] although its uptake seems to be unaffected by androgens.[5] For the purpose of clinical PET imaging, 11C-labelled acetate and 18F-labelled acetate are available. The multifarious biochemical functions of acetate explain the widespread biodistribution of these tracers in the heart, kidneys, liver, salivary glands, pancreas, spleen, intestine, bone marrow and skeletal muscle.
For the diagnosis of primary PCa, 11C-acetate PET/CT has been reported to be inferior to MRI, because its uptake in PCa is similar to its uptake in areas of benign prostatic hyperplasia.[21] For the detection of regional lymph node metastases, the findings from a meta-analysis indicated that the sensitivity and specificity of imaging with 11C-acetate were 73% and 79%, respectively.[22] In this same meta-analysis, investigators found that in the setting of PSA relapse after radical treatment, the tracer was highly specific (specificity, >90%) but lacked sensitivity (sensitivity, 68%).[22] The depiction of metastases at 11C-acetate PET/CT depends on the PSA level and may not be sufficient in patients with a PSA level less than 1–3 ng/mL.[23] 18F-acetate is excreted in the urine. The clinical value of 18F-acetate has not been evaluated so thoroughly.
ANTI-1-AMINO-3-(18F)-FLUOROCYCLOBUATE-1- CARBOXYLIC (18F-FACBC)
FACBC is a radiolabelled analogue of l-leucine for PET imaging. Its normal biodistribution includes intense hepatic and pancreatic uptake and moderate to mild uptake in the salivary and pituitary glands, the bowel and the bone marrow. The fact that l-leucine is preferentially metabolised in muscle cells explains why FACBC increasingly accumulates in muscle tissue with time.[24] Urinary excretion of FACBC is minimal, but cellular uptake is rapid. Thus, imaging can be performed before the tracer accumulates in the bladder, typically 3 min after application. The uptake of FACBC is not specific to PCa and might also be observed in benign prostatic hyperplasia, inflammation and benign tumours. 18F-FACBC PET/CT detected localised PCa with a sensitivity and specificity of 67% and 66%, localising dominant prostate tumours with a sensitivity of 90%. However, MRI was indispensable as the combined use of 18F-FACBC-PET/CT and mp-MRI yielded a positive predictive value of 82% for tumour localisation in the sector-based analysis. 18F-FACBC has been recently approved by the FDA for the detection of recurrent PCa.[25]
PROSTATE-SPECIFIC MEMBRANE ANTIGEN (PSMA)
PSMA, expression is 100-fold to 1000-fold higher in PCa than in other tissues, also known as (glutamate carboxypeptidase II), is a transmembrane glycoprotein that is found on prostate epithelial cells, the small intestine, renal tubular cells, celiac ganglia and salivary glands. The degree of PSMA expression is associated with the time to tumour progression and the probability of cancer relapse[26] and, thus, provides a rational target not only for diagnosis and monitoring but also for targeted therapy. Andro gens will downregulate the expression of PSMA, and it is more abundant on the surface of castration-resistant tumours.[27] PSMA expression appears to correlate with disease aggressiveness.[28] A variety of ligands targeting PSMA have been developed, but they all target the enzymatic portion of PSMA and, therefore, mimic the substrate that normally binds to PSMA. 68Ga is obtained from generators that are on site. Recent studies demonstrate the excellent performance of 68Ga-PSMA PET/CT compared to conventional imaging including PET with other tracers (e.g. 18F-Choline and 11C-Choline) with regard to local staging.[23] 68Ga-PSMA PET/CT had a high specificity of 98% and a moderate sensitivity of 56% for LN-detection.[29] 68Ga-PSMA has been mostly studied in Germany, whereas 18F-DCFPyL has been studied mostly in the United States. 68Ga-labelled PSMA HBED-CC (68Ga-PSMA) was developed in Germany and has been widely studied there. It consists of a targeting moiety and a chelate to which is added 68Ga. 68Ga-PSMA PET/CT has been reported to clearly improve the detection of lymph node metastases compared to morphological imaging for staging primary PCa [Figure 3].[21]
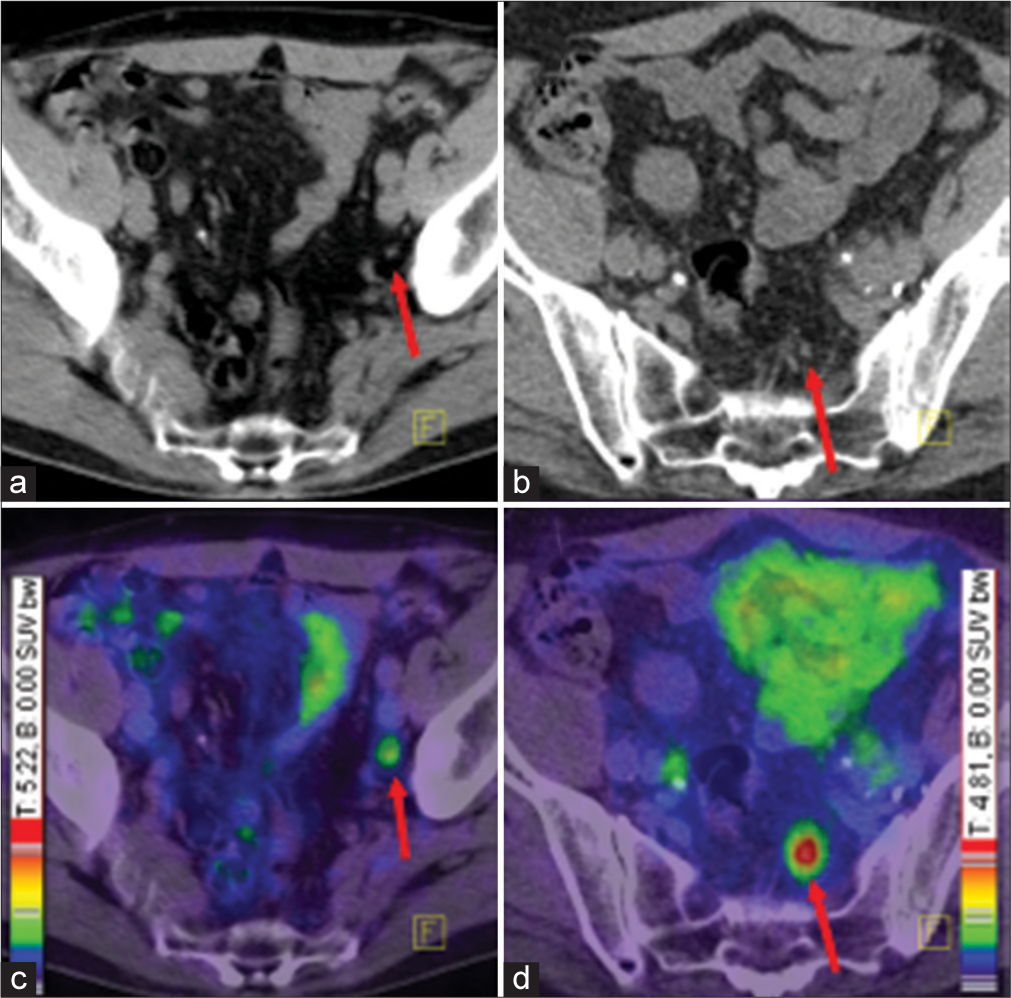
- Ga-68 PET CT demonstrating two different patients with small lymph node metastases and different intensity of tracer uptake. Tracer accumulation in lesions outside the prostate gland, should be regarded as prostate specific, until proven otherwise. Red arrows point to lymph node metastases. Colour scales were automatically produced by PET/CT machines. (a) CT of the first patient, (b) CT of the second patient, (c) fusion of PET and CT of the first patient, (d) fusion of PET CT of the second patient.
The true clinical value of PSMA PET/CT lies in its much higher sensitivity for recurrent disease. A recent meta-analysis involving several 68Ga-PSMA-11 PET articles covering 1309 BCR patients reported a summary sensitivity and specificity of 80% and 97%, respectively, on a per-lesion analysis. Even patients with very low levels of PSA were identified on 68Ga-PSMA-11 PET. In a retrospective study, Eiber et al.[30] studied 248 men with BCR using 68Ga-PSMA and detected a malignant lesion in 90%. Higher PSA levels and higher PSA velocity were correlated with higher tumour detection rates, but no significant association was seen with PSA doubling time. Interestingly, 68Ga-PSMA-11 PET/CT was more frequently positive in patients receiving androgen deprivation therapy (ADT) at the time of the scan than in patients without such treatment. 68Ga-PSMA (PSMA-based tracers) agent is most likely to become universally available in the clinic in the next few years due to its outstanding sensitivity and specificity for PCa and among all the novel radiotracers in this class.
FLUORINE-18-LABELLED SODIUM FLUORIDE (18F-NAF)
The radiolabelled form, 18F-NaF, was developed in 1962 and was approved for bone imaging by the U.S. Food and Drug Administration as early as 1972. Sodium fluoride (NaF) is an inorganic chemical compound that dissolves to give separated Na+ and F− ions. After chemisorption onto the bone matrix, 18F rapidly exchanges for the hydroxyapatite’s OH− ion, forming 18F-fluorapatite. Compared to diphosphonates, NaF has a more-rapid blood clearance and a higher bone-to-background ratio, and the interval from the administration to imaging is shorter. It is excreted by the kidneys into the urinary bladder.
It is used exclusively for imaging bone metastases and is not specific for PCa. It is mostly used in staging of high-risk cancers. 18F-NaF-PET/CT provides rapid, bone-specific uptake and excellent visualisation of the axial skeleton compared to technetium-99m methylene diphosphonate (99mTc-MDP) bone scan. However, 18F-NaF is not a comprehensive PET agent and is only capable of detecting bone metastases. In a recent prospective study, Apolo et al.[31] evaluated the ability of 18F-NaF-PET/CT to detect and monitor bone metastases over time in 60 patients with advanced PCa, who received primary definitive therapy. 18F-NaFPET/CT detected more bone metastases than 99mTc-based bone scans, in particular in patients with high metastatic risk without any known bone metastases on standard imaging (PSA of ≥10 ng/mL or a PSA doubling time of <6 months). For bone metastases, 18F-NaF compares favourably with other imaging agents [Figure 4]. Azad et al.[32] concluded that 18F-NaF, 11C-choline and 18F-choline PET/CT have equal sensitivities in finding PCa bone metastases although the specificity is higher for the choline-based agents.
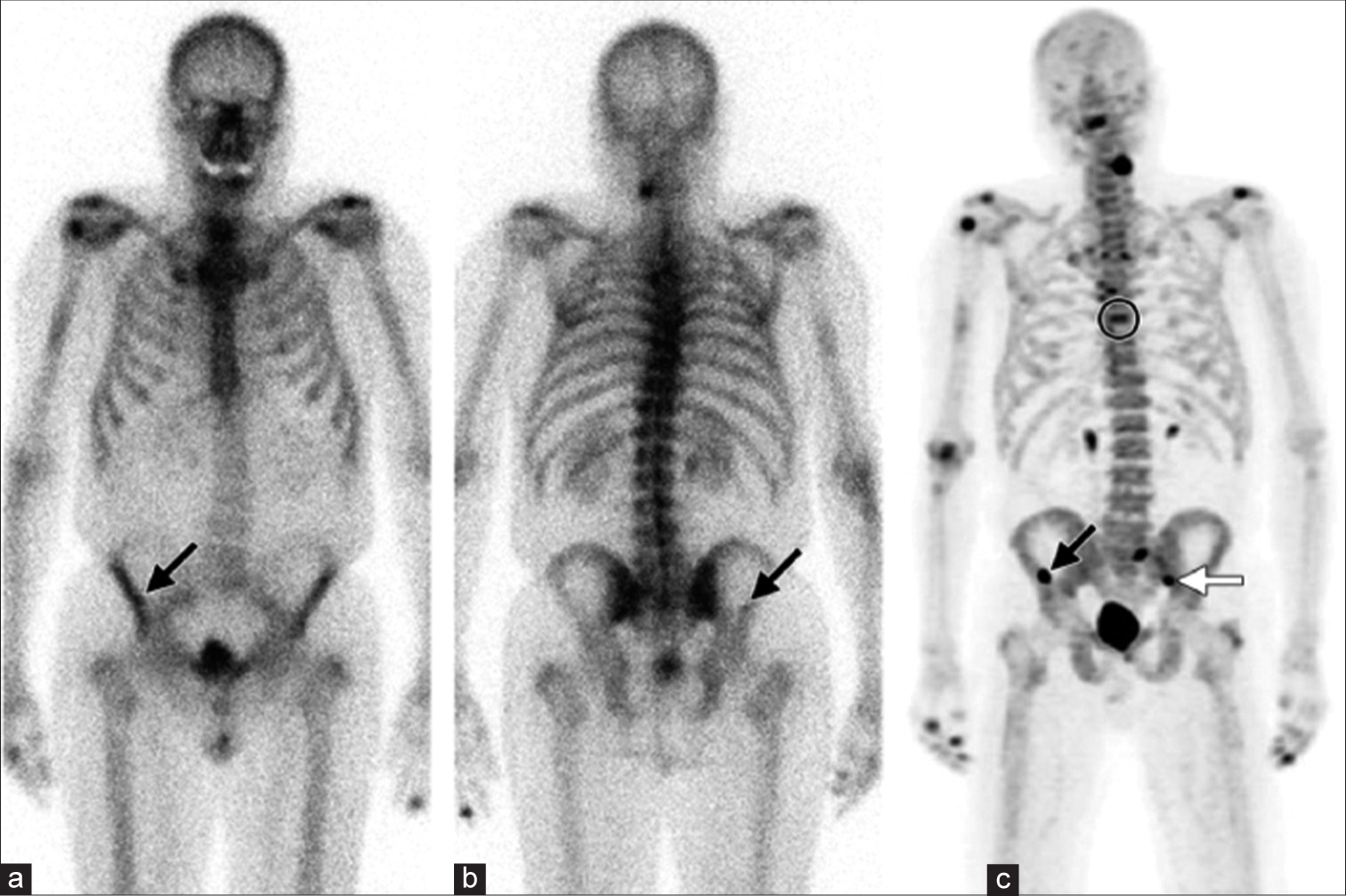
- Unsuspected bone metastases in a 77-year-old man with prostate carcinoma and a rising prostate-specific antigen level. (a and b) Anterior (a) and posterior (b) 99mTc-MDP bone scintigraphic images show a small area of suspicious uptake (arrow) in the right iliac wing. (c) Anterior 18F-NaF PET MIP image helps confirm the right iliac wing lesion (black arrow) and shows additional previously undetected bone lesions in theT7 vertebral body (circle) and the left sacroiliac joint (white arrow). PET: Positron emission tomography, 18F-NaF: Fluorine-18-labelled sodium fluoride, 99mTc-MDP: Technetium-99m methylene diphosphonate.
DIHYDROTESTOSTERONE ANALOGUES (18F-FDHT)
After intravenous administration, FDHT (structural analogue of 5a-dihydrotestosterone, the major and most potent intraprostatic androgen) is bound to sex hormone–binding globulin and passively diffuses through the cell membrane. In PCa, the uptake of FDHT reaches a plateau about 20 min after injection. The binding of FDHT to the androgen receptor (AR) is drastically reduced by physiologic testosterone levels and the administration[33] of antiandrogenic drugs ADT is thus, a first-line therapy for patients with advanced PCa eventually resulting in castration resistant prostate cancer (CRPC). FDHT has an effective half-life of 1–2 h and is mainly excreted by the liver by way of the biliary tree, as well as by the kidneys into the urinary bladder. Mild tracer uptake may also be observed in the pancreas, adrenals, intestine and the bone marrow. AR overexpression is present in the majority of CRPC patients indicating there are alternate pathways to activating the AR axis. AR-targeted imaging with PET can predict AR expression levels and consequently show the potential to image and assess the cancer, as well as to detect the therapeutic effect of AR-targeted drugs in specific patients. 18F-16β-fluoro-5α-dihydrotestosterone (18F-FDHT) is chemically similar to DHT. In a clinical trial conducted by Larson et al.,[33] which included patients with advanced aggressive PCa, 18F-FDHT showed lower sensitivity for PCa detection compared to 18F-FDG (86% vs. 97%, respectively). 18F-FDHT may be the better PET tracer for the assessment of treatment response[12] for in vivo estimation of the AR expression in patients on ADT. However, this radiotracer showed utility in the assessment of AR blockade with 2nd line anti-androgens.
BOMBESIN/GASTRIN-RELEASING PEPTIDE (GRP)
The membrane receptors of gastrin-releasing peptide receptor (GRPR) mediate their biologic effects through several intracellular signalling pathways, leading to an increased expression of transcription factors, key proteins of the cell cycle and expression of growth factor receptors. The gastrin-releasing peptide, also referred to as the ‘mammalian bombesin’, is a member of the ‘bombesin-like peptide family’ and shows a broad range of pharmacologic and biologic responses in mammalian organisms.
Two classes of GRP/bombesin analogues labelled with a variety of different PET radioisotopes have been developed, GRPR radio agonists and radio antagonists. Prior studies have shown high binding affinity of agonists to GRPRs, and their subsequent cell internalisation was believed to enhance the uptake, because the radioactivity was trapped in the intracellular compartment. GRPR was shown to be over-expressed in many cancer types, including PCa, while being scarce or non-detectable in normal prostate tissues [Figure 5]. GRP binds to 7-transmembrane G-protein coupled receptors which promotes tumour growth. Not all PCas express GRPR equally . De Visser et al.[34] reported low GRPR expression levels in poorly differentiated PCa. They are well-suited for both diagnostic and therapeutic purposes. However, other studies proved that antagonists are superior to agonists due to their considerably higher binding affinity to the GRPR. One of the most investigated radio antagonists for PCa imaging is statine-based JMV594 (DPhe-Gln-Trp-Ala-Val-Gly-His- Sta-Leu-NH2).[35] Moreover, labelling antagonists with 64Cu, 68Ga and 18F led to optimisation of pharmacokinetic properties. Overall, studies have shown the high efficiency of antagonists in the detection of primary PCa, while for bone metastases and BCR, the detection rates were much lower. More clinical data are needed on the use and efficacy of GRP targeted imaging in combination with PET in patients with PCa compared to PSMA-based agents.
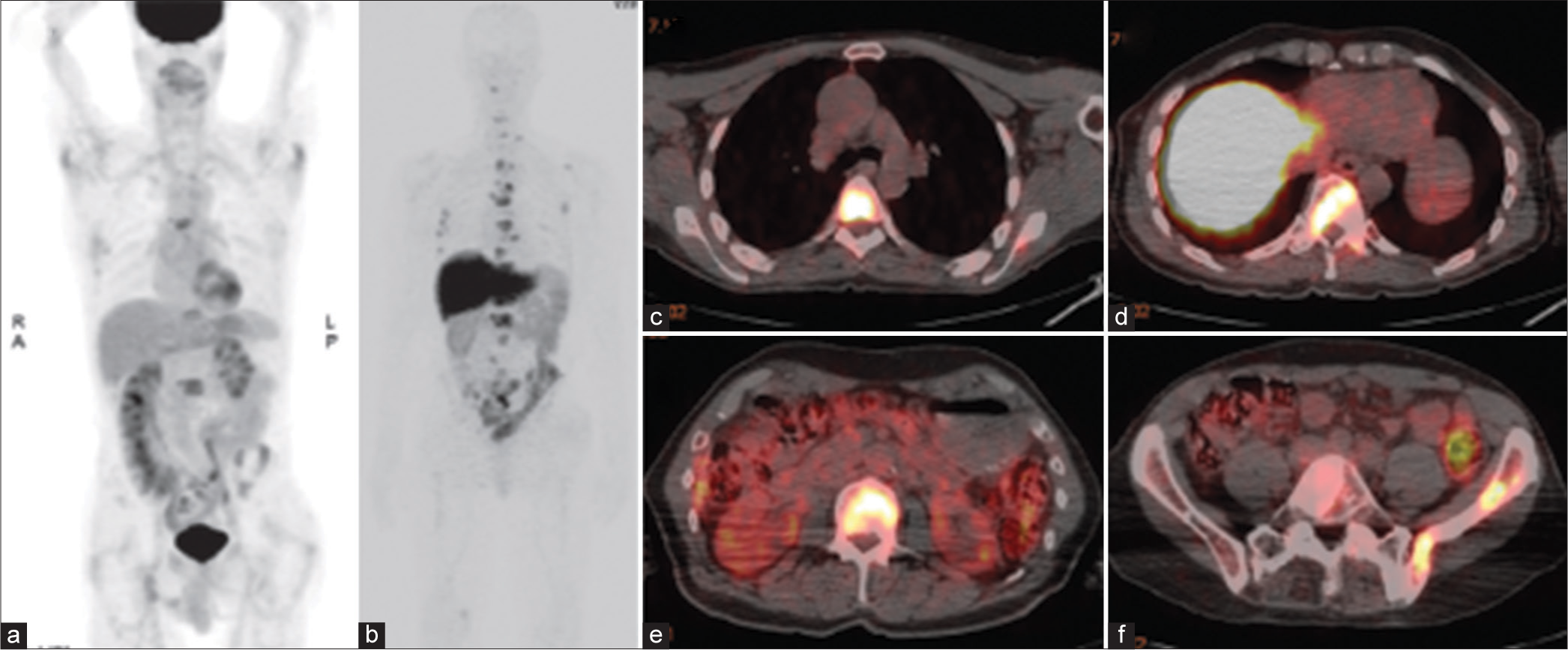
- FDG PET and 89zr-J591 PET/CT of a 73-year-old man with metastatic castration-resistant prostate cancer. (a) Coronal maximum intensity projection image from FDG PET shows a few metastases to bone. (b-f) Coronal maximum intensity projection image from 89Zr-J591 PET (b) and axial fused 89Zr-J591 PET/CT images (c-f) depict many more metastases to bone. FDG: 18F-fluorodeoxyglucose, PET: Positron emission tomography, CT: computed tomography.
IMAGING STRATEGIES
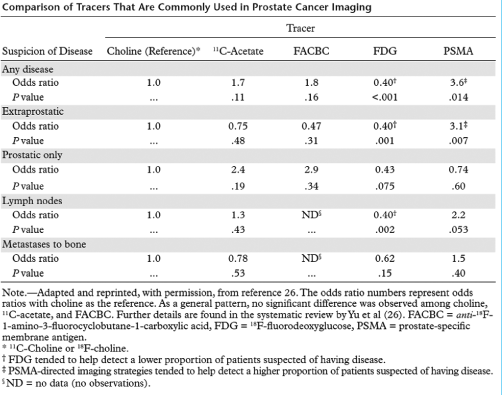
Table 2 summarises the currently available evidence about the diagnostic performance of the most commonly applied tracers for PCa imaging, according to a recently published systematic review.
 |
LOCAL STAGING
MRI is the most reliable modality for localisation of the tumour and assessment of its extent within and beyond the gland, including the detection of extracapsular tumour extension and invasion into the seminal vesicles for local staging of PCa. Diffusion-weighted MRI, MR spectroscopy and contrast material-enhanced MRI improve the diagnostic performance of conventional MRI and may also be used for non-invasive assessment of tumour aggressiveness.
LYMPH NODE STAGING
MRI has low sensitivity for the diagnosis of regional lymph node metastases but is the modality of choice for evaluating primary PCa. The findings from a meta-analysis, investigators reported a pooled sensitivity and specificity of 39% and 82%, respectively. 11C-Acetate may be more accurate for lymph node staging, because in the findings from a meta-analysis, investigators reported a pooled sensitivity of 73% and a pooled specificity of 79%.[22] For choline PET-CT, the results of a meta-analysis yielded a pooled sensitivity of 49% and a pooled specificity of 95%.[10] The potential of these agents to allow accurate assessment of the nodal status of PCa needs to be further evaluated, though PSMA-directed imaging agents have shown promising results in preliminary human studies.
RECURRENCE AFTER RADICAL TREATMENT
PSA is routinely measured to detect disease recurrence in patients who have undergone definitive treatment of localised disease and is a sensitive and specific tumour marker for PCa. Although PSA relapse after treatment is usually indicative of PCa recurrence, it does not contribute to identifying the sites of recurrent disease and the planning of targeted salvage therapy. The goal of imaging in this scenario is to distinguish local recurrence in the prostatic fossa from distant recurrence, most commonly in the lymph nodes or bones.
All sites of PCa recurrence
PCa recurrence for all sites, choline PET/CT was reported to have a high sensitivity (86%) and specificity (93%) in the findings from a meta-analysis, with 18F-choline performing slightly better than 11C-choline.[11] The pooled sensitivity of 11C-acetate for overall recurrence was reported to be 68%, with a pooled specificity of 93%.[22] Especially in patients after RP the systematic review suggested PSMA-directed tracers were reported to depict cancer foci in a higher proportion of patients with PSA relapse than did choline, acetate and FACBC.[26]
Local recurrence to the prostatic fossa
The findings from a meta-analysis disclosed values for pooled sensitivity and specificity of 82% and 87% after RP and values of 82% and 74%, respectively, after external beam radiation, for the diagnosis of local recurrence to the prostatic fossa with MRI. The diagnostic precision of 11C-acetate in this setting strongly depends on the patient’s PSA level and is higher after RP than after RT. Overall, the pooled sensitivity for 11C-acetate PET/CT was reported to be 83% and its sensitivity was 92% in the findings from a meta-analysis.[22]
In the results of one study that directly compared MRI and 18F-choline PET/CT for the detection of local recurrence after RP, investigators found that MRI had better diagnostic accuracy, especially in patients with low levels of PSA. For choline PET/CT, the pooled sensitivity was calculated to be 75%, and the pooled specificity was 82%.[11] In the results of a systematic comparison of choline, acetate, FACBC and PSMA-directed tracers, investigators did not find significant differences in the detection of recurrence in the prostatic fossa.[26]
PCa recurrence in regional lymph nodes
Choline PET/CT was reported to be perfectly sensitive in the results of a meta-analysis, with a pooled specificity of 82%,[11] and 11C-acetate had a pooled sensitivity and specificity of 82% and 94%, respectively[22] with regard to recurrence in regional lymph nodes. PSMA-directed tracers may help detect lymph node metastases in a higher proportion of patients than acetate and choline.[26] In the setting of PSA relapse, although more data are needed to verify these preliminary observations.
Metastases to bone
99mTc-based bone scintigraphy is the most widely distributed imaging method for the detection and localisation of metastases to bone in PCa patients. Although it offers the advantages of broad availability and low cost but comes with a relatively low diagnostic accuracy, since it has low resolution a recent meta-analysis showed that choline-based PET/CT studies are more sensitive than conventional bone scans in the findings.[12] In patients with primary intermediate- or high-risk PCa or in those with biochemical relapse and serum PSA levels of more than 2.0 ng/mL,[15] 18F-acetate PET/CT is a valuable tool for helping detect metastases to bone. The results of a systematic review of 18F-NaF and choline-based PET/CT, investigators found similar diagnostic performances for both modalities. Another emerging imaging approach for patients at high risk for metastases to bone from PCa is whole-body MRI, which was shown to outperform conventional bone scans and had a lower sensitivity but higher specificity than 18F-NaF PET/CT in recent studies. To conclude, although the diagnostic accuracy of choline-based PET/CT, acetate-based PET/CT and NaF-based PET/CT is not perfect, they are the most sensitive, specific and adequately evaluated imaging modalities for metastases to bone from PCa at the current time. Emerging imaging techniques and tracers (FACBC, PSMA-directed agents, etc.) are undergoing preclinical and clinical evaluation and may further enhance diagnostic precision in the assessment of patients with PCa recurrence. There is an absence of a standard methodologic approach in evaluating metastatic PCa of newly developed compounds, which limits the comparability of reported results among different tracers.[36]
CONCLUSION
18F-FDG performs poorly in PCa numerous PET agents have been developed to identify primary tumours, stage them, detect recurrence after treatment and monitor metastases. Early diagnosis and accurate staging of clinically significant PCas are the most pivotal factors determining outcome. The clinical use of these agents in PCa is being investigated; however, it is very difficult to compare different agents as patient populations and scanner variability limit comparisons, and it is difficult to combine 2 or more PET agents in one study due to the expense and radiation exposure. Despite the considerable limitations, PET seems to be useful for the diagnosis and staging of known or suspected primary PCa with high Gleason scores, in the detection of BCR in locally recurrent or metastatic disease in patients with rising PSA levels, in monitoring response to therapies and in prognostication. PSMA PET/CT has shown unchallengeable results in PC both in the initial setting and biochemical failure. Irrespective of PSA level, PSMA PET/CT outperforms CT and BS combined. However, most radiolabelled agents are still in the early stage of clinical evaluation, and therefore, it is difficult to comment on which agent is the most useful for imaging of primary disease and risk stratification.
18F-FDG, the most common PET radiotracer, is generally limited in the diagnosis and staging of clinically organ-confined PCa; however, it may be able to indicate the aggressiveness of disease. Similarly, radiolabelled acetate and choline tracers, as well as 18F-FACBC are equally useful in imaging locally recurrent or metastatic disease in men with biochemical relapse but show marked limitations in their ability to detect primary PCa. PSMA is a more sensitive and specific radiotracer with a high sensitivity for early recurrent disease. Finally, GRP-targeted PET imaging has shown high efficiency in the detection of primary PCa, while the sensitivity for bone metastases and BCR was much lower. Dihydrotestosterone analogues have a limited role in detecting AR and the effects of androgen blockage. On the other hand, in detecting occult bone metastases, 18F-NaF showed superiority, compared to standard imaging, in high-risk patients with PCa particularly. Still, more clinical data are needed to prove the efficacy of these PET tracers. The development of other novel radiopharmaceuticals is expected in the future, as it is clear that the ideal agent has not yet been developed; thereby, additional studies are required for existing PET.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- SEER stat fact sheets: Prostate cancer-statistics at a glance. 2015. National Cancer Institute Web Site. Available from: https://seer.cancer.gov/statfacts/html/prost.html [Last accessed on 2015 Jan 12]
- [Google Scholar]
- Molecular pathways in prostate cancer. Nephrourol Mon. 2013;5:792-800.
- [CrossRef] [PubMed] [Google Scholar]
- The high prevalence of undiagnosed prostate cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int J Cancer. 2015;137:2795-802.
- [CrossRef] [PubMed] [Google Scholar]
- Causespecific mortality following radical prostatectomy. Prostate Cancer Prostatic Dis. 2012;15:106-10.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632-42.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of hypoxia on the uptake of [methyl-3H] choline, [1-14C] acetate and [18F] FDG in cultured prostate cancer cells. Nucl Med Biol. 2006;33:977-84.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical patterns of recurrence following biochemical relapse in the dose escalation era for prostate patients undergoing external beam radiotherapy. J Urol. 2015;194:1624-30.
- [CrossRef] [PubMed] [Google Scholar]
- The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67:1009-16.
- [CrossRef] [PubMed] [Google Scholar]
- Multiparametric magnetic resonance imaging of recurrent prostate cancer. Top Magn Reson Imaging. 2016;25:139-47.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular imaging of prostate cancer: PET radiotracers. AJR Am J Roentgenol. 2012;199:278-91.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of prostate cancer with PET/CT using 18F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5:96-108.
- [Google Scholar]
- Advancements in MR imaging of the prostate: From diagnosis to interventions. Radiographics. 2011;31:677-703.
- [CrossRef] [PubMed] [Google Scholar]
- Hallmarks of cancer: The next generation. Cell. 2011;144:646-74.
- [CrossRef] [PubMed] [Google Scholar]
- Dual-modality imaging: Combining anatomy and function. J Nucl Med. 2008;49:938-55.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51:1511-20. discussion 1520-1
- [CrossRef] [PubMed] [Google Scholar]
- Technical limits of PET/CT with 18FDG in prostate cancer. Aktuelle Urol. 2006;37:218-21.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317-23.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical advances of fluorine-2-Ddeoxyglucose--positron emission tomography/computed tomography in urological cancers. Int J Urol. 2010;17:501-11.
- [CrossRef] [PubMed] [Google Scholar]
- AcetylCoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287-94.
- [CrossRef] [PubMed] [Google Scholar]
- 1-11C-Acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med. 2008;49:327-34.
- [CrossRef] [PubMed] [Google Scholar]
- 11C-Acetate PET/CT in localized prostate cancer: A study with MRI and histopathologic correlation. J Nucl Med. 2012;53:538-45.
- [CrossRef] [PubMed] [Google Scholar]
- Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU Int. 2013;112:1062-72.
- [CrossRef] [PubMed] [Google Scholar]
- 11C-Acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med. 2003;44:549-55.
- [Google Scholar]
- Expression of glucose transporter 1 (Glut-1) in cell lines and clinical specimens from human prostate adenocarcinoma. Anticancer Res. 2004;24:3057-63.
- [Google Scholar]
- Assessment of PET tracer uptake in hormone-independent and hormone-dependent xenograft prostate cancer mouse models. J Nucl Med. 2011;52:1654-63.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative performance of PET tracers in biochemical recurrence of prostate cancer: A critical analysis of literature. Am J Nucl Med Mol Imaging. 2014;4:580-601.
- [Google Scholar]
- Bone metastases in castration-resistant prostate cancer: Associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology. 2014;271:220-9.
- [CrossRef] [PubMed] [Google Scholar]
- Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195-201.
- [CrossRef] [PubMed] [Google Scholar]
- C11-acetate and F-18 FDG PET for men with prostate cancer bone metastases: Relative findings and response to therapy. Clin Nucl Med. 2011;36:192-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate-Specific Membrane Antigen Ligands for Imaging and Therapy. J Nucl Med. 2017;58:67S-76S.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective study evaluating Na F18 PET CT in predicting clinical outcomes and survival in advanced prostate cancer. J Nucl Med. 2016;57:886-92.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular and functional imaging of bone metastases in breast and prostate cancers: An overview. Clin Nucl Med. 2016;41:e44-50.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366-73.
- [Google Scholar]
- ProCA1, GRPR: A new imaging agent in cancer detection. Biomark Med. 2016;10:449-52.
- [CrossRef] [PubMed] [Google Scholar]
- Review of commonly used prostate specific PET tracers used in prostate cancer imaging in current clinical practice. Clin Imaging. 2021;79:278-88.
- [CrossRef] [PubMed] [Google Scholar]
- Perspectives on mTOR inhibitors for castration-refractory prostate cancer. Curr Cancer Drug Targets. 2012;12:940-9.
- [CrossRef] [PubMed] [Google Scholar]






